MENU
SERVICES
Hours
Monday : 10am – 7pm
Tuesday: 10am – 7pm
wednesday: 10am – 7pm
thursday: 10am – 7pm
friday: 10am – 7pm
Saturday: 10am – 7pm
Sunday: 10am – 5pm
(Closed for Lunch)
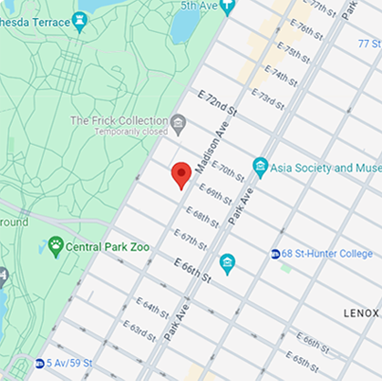
Location
818 Madison Ave, 3rd Floor
New York, NY 10065
3rd floor accessible by elevator or stairs
MedSpa Marketing Powered by Branding New York City
© Beauté Aesthetics New York 2024. All Rights Reserved.




